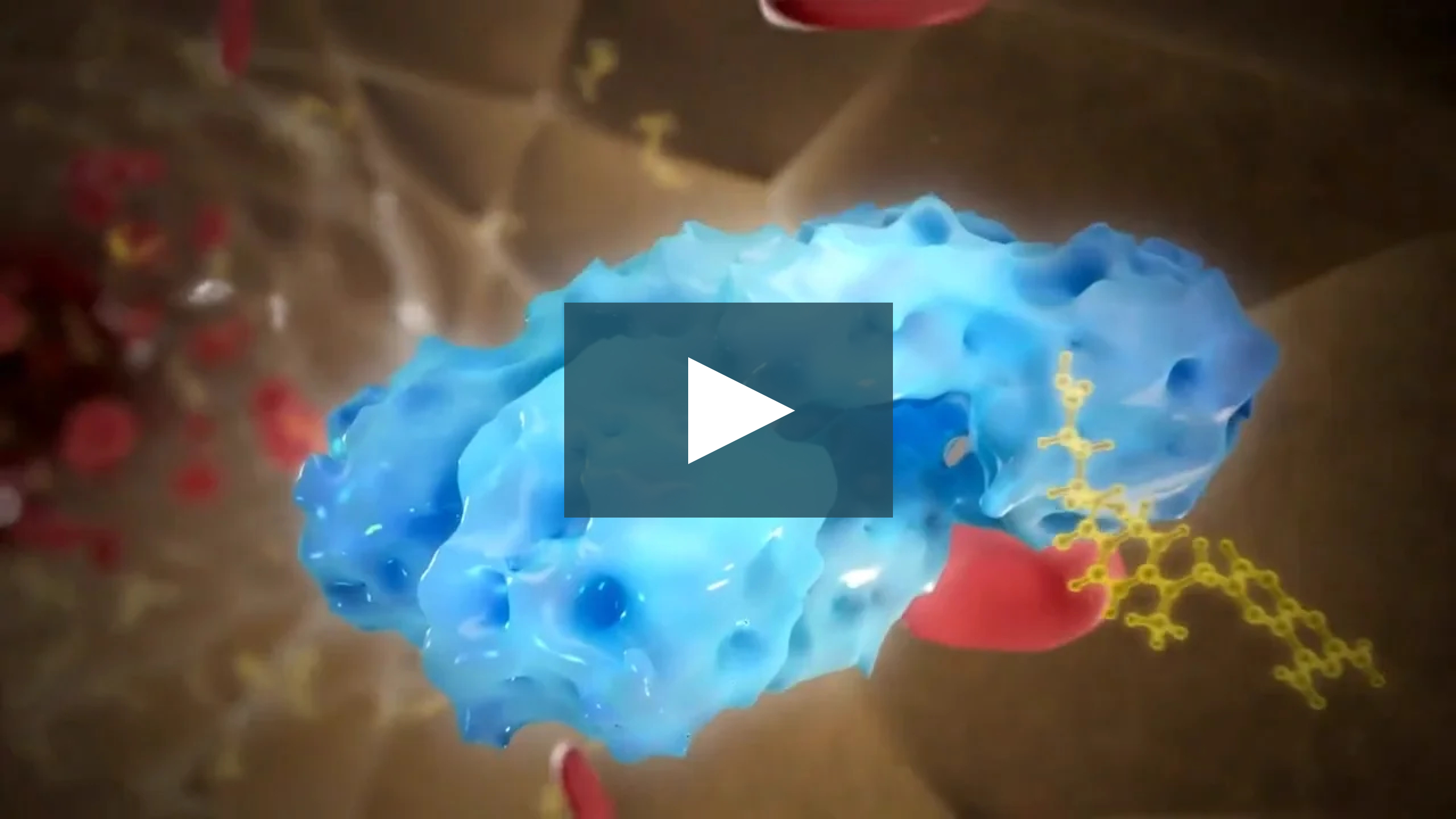
MECHANISM OF ACTION

Voraxaze Works in the Circulation to Quickly Clear Methotrexate (MTX)1,2
Voraxaze and Leucovorin Work Differently, Yet Complementarily1,2
Voraxaze® (glucarpidase) lowers toxic levels in the extracellular compartment, whereas leucovorin counteracts MTX intracellularly. Only Voraxaze rapidly reduces plasma MTX concentrations by hydrolyzing extracellular MTX.1,2
When MTX concentrations are too high, no plasma leucovorin concentration may be sufficient to ensure effective cell rescue.2
Voraxaze rapidly cleaves MTX into 2 inactive metabolites to provide a nonrenal pathway for elimination.1,3
Leucovorin Rescue Is Essential, But It Does Not Clear MTX From the Body2,4
- Leucovorin provides intracellular rescue, restoring tetrahydrofolate stores and allowing resumption of DNA and RNA synthesis, but it does not clear MTX from the body.2,3,4
- Leucovorin is less effective in the presence of high circulating MTX concentrations, particularly MTX concentrations exceeding 10 µM for ≥48 hours.3
- Leucovorin is a storage vitamin, and excessive doses may impact future high-dose methotrexate (HDMTX) treatment courses. HDMTX treatment failures have been reported in pediatric patients following high doses of leucovorin.3