Resources

Methotrexate (MTX) Monitoring Tool
MTXPK.org
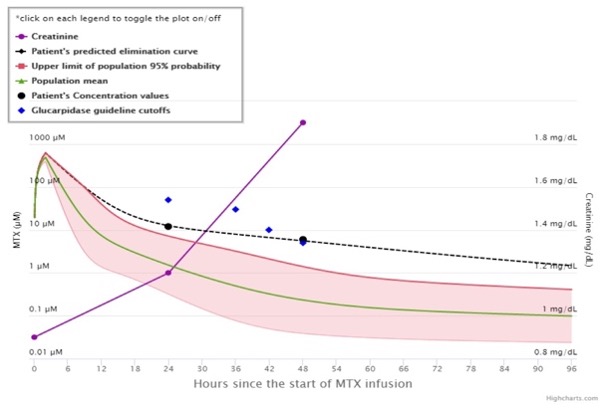
MTXPK.org is a powerful tool that helps you monitor how well your patients are clearing MTX. MTXPK.org can be used to determine when Voraxaze® (glucarpidase) is indicated for your patients and you can get started with a single MTX and serum creatinine (SCr) lab value1,2:
- Know when to expect your patient’s MTX concentration to reach a level acceptable for discharge
- Evaluate your patient’s expected MTX clearance
- Compare your patient’s MTX clearance with the population mean and 2 SD above the mean
- See how your patient’s MTX levels compare with levels known to confer risk for serious MTX toxicity based on expert consensus guidelines
- Evaluate SCr trend
- Anticipate and diagnose delayed MTX clearance early, allowing you to act within the critical treatment window of 48-60 hours to lower circulating MTX levels and facilitate renal recovery1,3,4
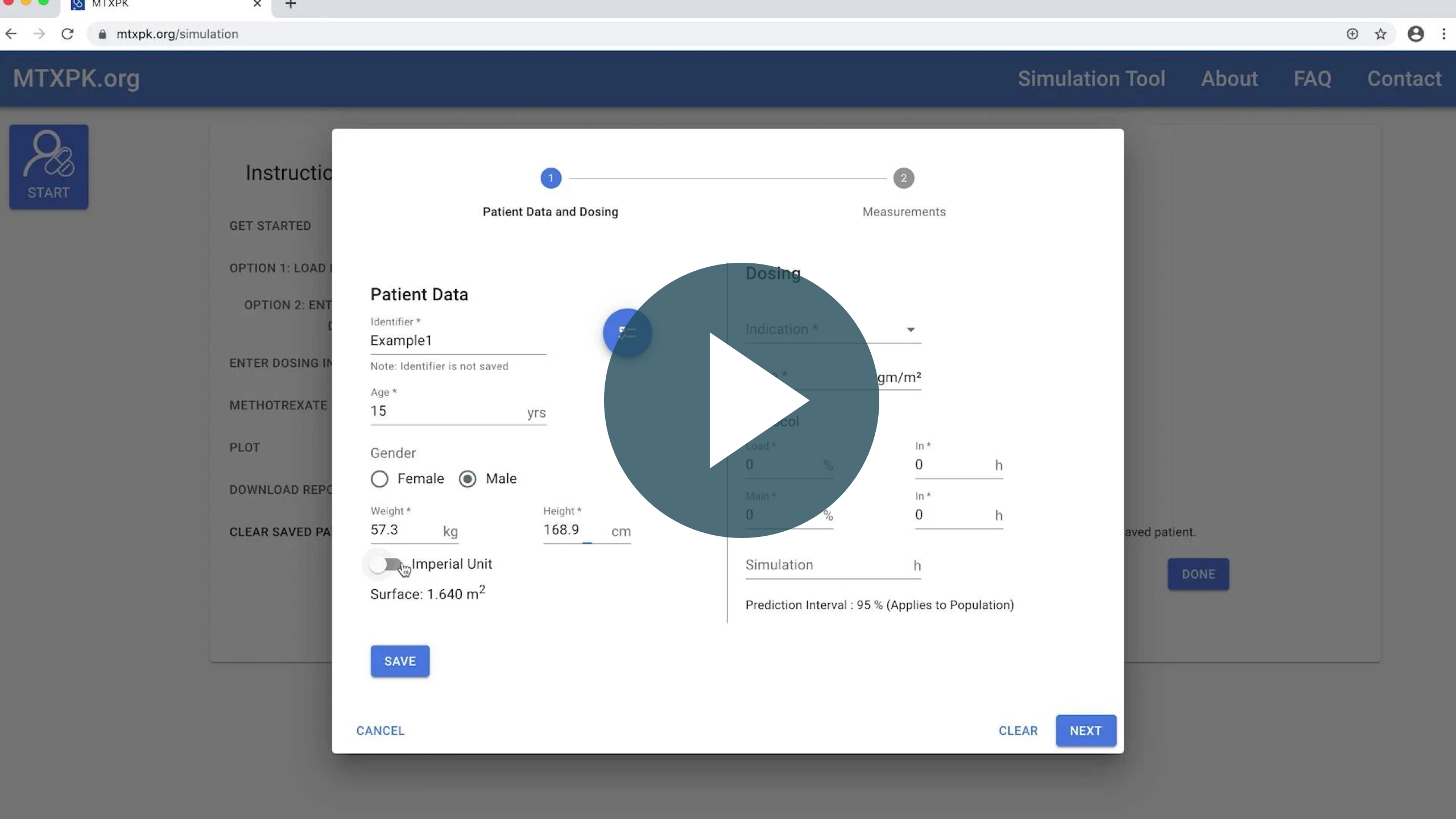
MTXPK Tutorial Video
A brief tutorial that walks you step by step through the process of using MTXPK.org to monitor your patients’ methotrexate (MTX) clearance.
MTXPK.org - Background, Overview and Applications
In partnership with the HOPA Industry Relations Council, please check out this recorded “Ask the Experts” interactive discussion on MTXPK.org!
In this recorded session, you will hear from Laura Ramsey, PhD, the creator of MTXPK.org and primary author of the Consensus Guidelines, as well as Jill Lykon, PharmD, BCOP, a clinical hematology/oncology pharmacy coordinator from the University of Miami Hospital and Clinics who reviews practical applications of MTXPK.org.

MTXPK.org Clinical Applications Leave-Behind
This quick resource provides you with an overview of MTXPK.org, including key features and benefits of using MTXPK.org to monitor how well your patients are clearing methotrexate (MTX).

MTXPK.org: A Clinical Decision Support Tool Evaluating High‐Dose Methotrexate Pharmacokinetics to Inform Post‐Infusion Care and Use of Glucarpidase
Overview of the web-based clinical decision support tool, MTXPK.org, that helps clinicians monitor whether patients are clearing methotrexate (MTX) as expected.
Guidelines

Consensus Guidelines for Voraxaze® (glucarpidase) Use
Consensus Guideline for Use of Glucarpidase in Patients With High‐Dose Methotrexate Induced Acute Kidney Injury and Delayed Methotrexate Clearance.

Highlights - Consensus Guidelines
Highlighted excerpts from the Consensus Guideline for Use of Glucarpidase in Patients With High-Dose Methotrexate Induced Acute Kidney Injury and Delayed Methotrexate Clearance.

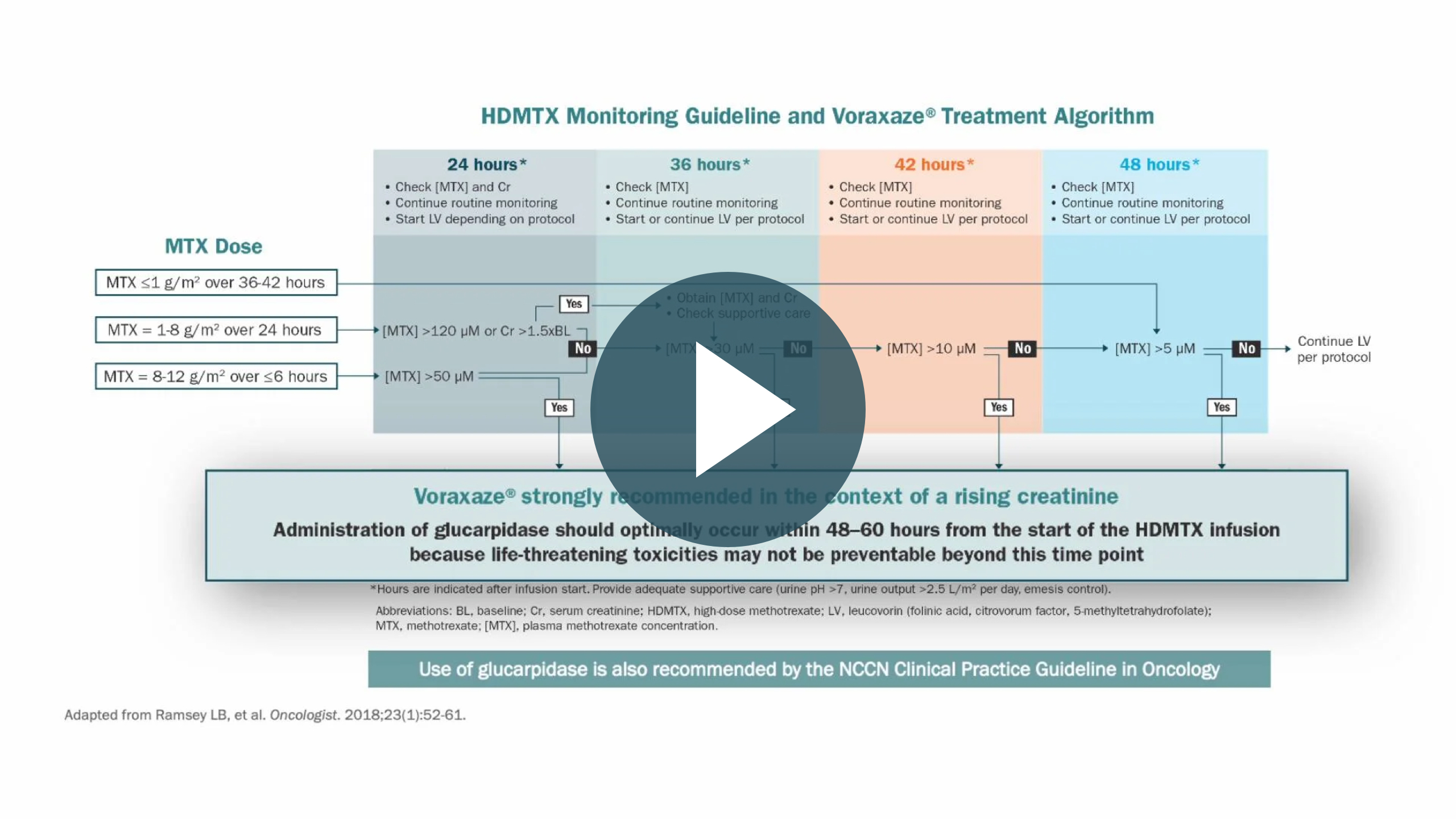
Voraxaze® (glucarpidase) Treatment Algorithm
Download and print for your practice: Treatment algorithm based on the Consensus Guidelines.

Expert Consensus Stocking Guidelines
Review the stocking guidelines established by an international expert panel.
Educational and Downloadable Tools
Early Warning Signs of Delayed Methotrexate (MTX) Clearance
Download this resource to help you identify the early warning signs of delayed MTX clearance.
Voraxaze 5-Minute Educational Video
Watch and learn about the importance of identifying delayed methotrexate (MTX) clearance and acute kidney injury early, and when to use Voraxaze® (glucarpidase).

HDMTX Roadmap for Success
Download this resource to guide you through successful management of delayed methotrexate (MTX) clearance in patients being treated with high-dose MTX (HDMTX).
Patient Testimonials
Matthew’s Story: A Race Against the Clock
Hear Matthew’s story of how Voraxaze was ordered, delivered, and administered in his time of need.
Matthew’s Story: Living Life Cancer-Free
Hear Matthew’s story, an osteosarcoma survivor who received Voraxaze during his cancer treatment. Matthew successfully completed his treatments and paved the way for a cancer-free life.
HCP Testimonials
Mitigating Methotrexate Toxicity With Voraxaze in Osteosarcoma
Hear Dr. Matteo Trucco discuss the importance and risks of methotrexate in MAP chemotherapy and how Voraxaze effectively counters methotrexate toxicity, preventing further toxicity and ensuring patient safety.
Completing MAP Chemotherapy and the Essential Role of Voraxaze
Discover Dr. Kurt Weiss’ insights on the importance of completing MAP chemotherapy with consideration to surgery with osteosarcoma patients and the vital role of Voraxaze in treating methotrexate toxicity.
Expert Discussions
OncLive® Webinar
Watch leading experts discuss the current landscape of high-dose methotrexate (HDMTX) toxicity and treatment options to improve patient outcomes.
Consensus Guideline Expert Interview
Watch and listen to experts discuss important information about guidelines for managing patients with delayed methotrexate (MTX) clearance due to renal impairment.
Clinical Articles
Annals of Emergency Medicine
Expert Consensus Guidelines for Stocking of Antidotes in Hospitals That Provide Emergency Care
Dart RC et al, 2018.
Review recommendations of an expert panel for the stocking of antidotes, including Voraxaze® (glucarpidase), in emergency departments.
Cancer
Resumption of High-Dose Methotrexate After Acute Kidney Injury and Glucarpidase Use in Pediatric Oncology Patients
Christensen AM et al, 2012.
The authors report on clinical data and outcomes in pediatric patients who received Voraxaze® (glucarpidase) after treatment with high-dose methotrexate (HDMTX).
High-Dose Methotrexate-Induced Nephrotoxicity in Patients With Osteosarcoma
Widemann BC et al, 2004.
This review of trials in patients with osteosarcoma and high-dose methotrexate (HDMTX)-induced acute kidney injury compares the efficacy of dialysis-based methods and time to renal recovery after supportive treatment with efficacy and renal recovery after treatment with Voraxaze® (glucarpidase).
Clinical Journal of Oncology Nursing
High-Dose Methotrexate: Nurse Considerations for Administration and Supportive Care
Roche et al., 2023.
This article provides an overview of High-Dose Methotrexate (HDMTX) treatment and nursing considerations, including HDMTX toxicities, components of supportive care, and strategies to manage HDMTX administration and delayed elimination.
Clinical Pharmacology & Therapeutics
MTXPK.org: A Clinical Decision Support Tool Evaluating High-Dose Methotrexate Pharmacokinetics to Inform Post-Infusion Care and Use of Glucarpidase
Taylor ZL et al, 2020.
Overview of a web-based clinical decision support tool that helps clinicians monitor whether patients are clearing methotrexate (MTX) as expected.
Clinical Toxicology
Pooled Analysis of Time to Administration of Glucarpidase for Methotrexate Toxicity Versus Mortality
Ward S et al, 2013.
A pooled analysis of data from 476 patients with high-dose methotrexate (HDMTX)-induced renal toxicity and delayed MTX clearance who received treatment with Voraxaze® (glucarpidase) that examines overall mortality based on days between start of HDMTX infusion and first Voraxaze dose. (Abstract #7)
ClinicoEconomics and Outcomes Research
Glucarpidase for Treating Adults with Delayed Methotrexate Elimination Due to Impaired Renal Function: An Economic Simulation Analysis
Kala JK, et al., 2023
A decision tree model was developed to assess the economic impact of glucarpidase. Treatment of all eligible patients with glucarpidase within 60 hours was associated with an increased cost per patient (relative to current practice) but substantial improvements in clinical outcomes. Timely glucarpidase use was less expensive than delayed glucarpidase or hemodialysis.
Length of Stay, Mortality, and Readmissions Among Medicare Cancer Patients Treated With Glucarpidase and Conventional Care: A Retrospective Study
Demiralp B et al, 2019.
Examines hospital length of stay, mortality, and readmission rates for Medicare cancer patients with delayed clearance of methotrexate (MTX) treated with Voraxaze® (glucarpidase).
Journal of Clinical Oncology
Glucarpidase, Leucovorin, and Thymidine for High-Dose Methotrexate-Induced Renal Dysfunction: Clinical and Pharmacologic Factors Affecting Outcomes
Widemann BC et al, 2010.
An analysis of the role of Voraxaze® (glucarpidase), leucovorin, and thymidine in the management and outcomes of patients with high-dose methotrexate (HDMTX)-induced renal dysfunction, including factors associated with development of Grade 4 or fatal toxicity in these patients.
High-Dose Methotrexate in Patients With Lymphoma: Predictors of a Complicated Course
O’Donoghue et al., 2022
This retrospective analysis comprised of 642 adult patients with lymphoma with 2,804 cycles of HDMTX. The incidence of AKI was 19.1% with AKI grade ≥ 2 making up 21% of cases. Rates of AKI, ICU admission, and 30-day mortality are associated with elevated 48-hour MTX levels. There was a significant increase in median LOS with elevated MTX levels (P < .001). Receiver operator characteristic curve analysis for AKI grade ≥ 2 demonstrated a 48-hour MTX level threshold of 1.28 μmol/L. Multivariate logistic regression analysis revealed age, male sex, elevated body surface area, higher MTX dose, monotherapy, and first cycle as independent factors.
Journal of Onco-Nephrology
Acute kidney injury associated with high dose methotrexate
Mamlouk O., et al., 2019
A 46 year-old man with high-grade B cell lymphoma was admitted for treatment with rituximab and high dose methotrexate (MTX). The patient was started on intravenous (IV) fluid with sodium bicarbonate for volume expansion and alkalization of urine, then 3.5 g/m2 of MTX was infused over 4 h. Serum creatinine (SCr) prior to infusion was of 1.1 mg/dL and increased to 1.24 and 4.5 mg/dL at 12 and 36 h after the infusion, respectively. The patient was diagnosed with methotrexate-induced crystalline nephropathy. This article shows images of MTX crystals from the urine of affected patients that show its detailed structure.
Pediatric Blood & Cancer
Delayed Elimination of High-Dose Methotrexate and Use of Carboxypeptidase G2 in Pediatric Patients During Treatment for Acute Lymphoblastic Leukemia
Svahn T et al, 2016.
A report on pediatric patients with acute lymphoblastic leukemia who were treated between 2008–2014 according to the Nordic Organization for Pediatric Hematology and Oncology (NOPHO) ALL 2008 protocol, which includes administration of 6-8 high-dose methotrexate (HDMTX) courses as well as recommendations for intervention with Voraxaze® (glucarpidase) in the presence of delayed MTX clearance.
Methotrexate Level Discrepancy Post-Glucarpidase: A Pediatric Case Series and Review of Literature
Kibby D, Trinkman H, 2024.
Laboratory interference with immunoassay measurement post-glucarpidase administration is well established, with current product labeling indicating this persists for 48 h. However, recent experience in pediatric patients supports this discrepancy persists beyond 48 h. Three cases experienced delayed methotrexate clearance and received glucarpidase with subsequent measurement of methotrexate levels by liquid chromatography tandem mass spectrometry (LC‑MS/MS) and/or immunoassay. Within this case series, discrepancies between LC‑MS/MS and immunoassay levels persisted significantly longer than 48 h.
The Oncologist
Consensus Guideline for Use of Glucarpidase in Patients With High-Dose Methotrexate Induced Acute Kidney Injury and Delayed Methotrexate Clearance
Ramsey LB et al, 2018.
Expert Consensus Guidelines for the timely and effective use of Voraxaze® (glucarpidase) to treat high-dose methotrexate (HDMTX)-induced acute kidney injury and delayed MTX clearance, including supportive care and MTX monitoring before and after treatment.
Preventing and Managing Toxicities of High-Dose Methotrexate
Howard SC et al, 2016.
An overview of the literature regarding high-dose methotrexate (HDMTX) therapy and risk for associated toxicities – including comprehensive recommendations for prevention and management of toxicity and treatment guidance for HDMTX-induced acute kidney injury.
Glucarpidase (Carboxypeptidase G2) Intervention in Adult and Elderly Cancer Patients With Renal Dysfunction and Delayed Methotrexate Elimination After High-Dose Methotrexate Therapy
Schwartz S et al, 2007.
A review of the potential risks involved in cancer treatment with high-dose methotrexate (HDMTX), including acute kidney injury and delayed MTX clearance, and evaluation of rescue intervention with Voraxaze® (glucarpidase).
Understanding and Managing Methotrexate Nephrotoxicity
Widemann BC et al, 2006.
Review the pharmacology of methotrexate (MTX), incidence of high-dose MTX–induced renal impairment, and various approaches to treatment, including conventional supportive care and leucovorin rescue, dialysis-based methods, and Voraxaze® (glucarpidase).